Home | Quality Assurance Program
Modern Aminos Quality Assurance Program
Modern Aminos Quality Assurance Program
Verifying Your Product: Understanding the COA and Batch Number
Every product at Modern Aminos includes both a Batch Number and a QR Code on the vial label.
These identifiers allow you to independently confirm that your vial matches its verified laboratory report.
Here’s how to verify your product step-by-step:
1: Scan the QR Code on your vial label — it will take you directly to the specific Certificate of Analysis (COA) for that batch. If for some reason the QR code is not working you can match the coa to the label using the unique number above the QR code.

2: Match the Batch Number on your vial to the batch number printed on the COA and vial photo.
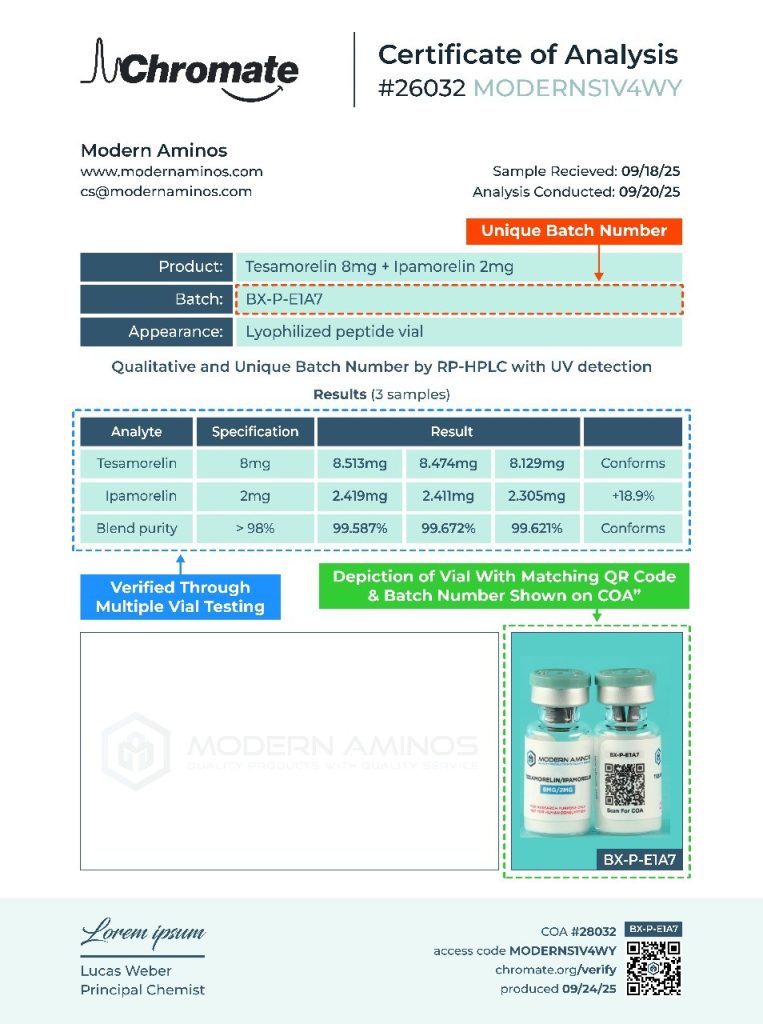
Review the Testing Results to confirm purity, identity, and quantity metrics.
This ensures the product you’re holding was independently tested and verified before release , providing transparency, authenticity, and confidence in your research materials.
Our Commitment to Quality
At Modern Aminos, we’re proud to uphold the Modern Aminos Quality Assurance Program, our ongoing promise to deliver unmatched purity, accuracy, and consistency in every research product.
This program represents our dedication to transparency, scientific integrity, and excellence, ensuring that every batch we release is independently tested, verified, and traceable.
Our Handling of Research Chemicals
Before any product is shipped, it’s handled under strict quality control protocols designed to preserve stability, purity, and accuracy.
- All materials are stored in sanitary, temperature-controlled, and secure environments, protected from tampering, light, and microbial exposure.
- Samples are collected and analyzed by independent third-party laboratories prior to batch approval.
Any batch that does not meet our strict quality standards is rejected and discarded with no exceptions.
Receiving & Sampling Procedures
When new raw materials arrive at Modern Aminos, they are not placed directly into active inventory.
Instead, each shipment is stored in a quarantine zone, physically separated from approved inventory.
Only trained quality personnel may access this area to collect samples for testing.
When multiple containers of the same product arrive, samples from each container are combined into a composite sample to ensure testing reflects the entire batch, not just one portion.
The composite sample is submitted to one of our trusted third-party labs — such as Vanguard, Chromate, or Freedom Diagnostics — where it undergoes a tailored testing panel specific to its molecular structure.
No raw material is approved for packaging or sale until all testing confirms full compliance with Modern Aminos standards. Any failed batch is immediately rejected and discarded.
Our Laboratory Partners
We only work with established, accredited testing facilities that meet or exceed regulatory standards.
Our current partners include:
Vanguard Laboratory
Chromate
TrustPointe Analytics
Freedom Diagnostics
If you have any questions regarding their process we highly recommend you visit one of their websites and reach out to them!
All Certificates of Analysis (COAs) are directly linked to these original lab reports for full transparency.
If you ever wish to verify a COA’s authenticity, you may contact the testing lab directly using the information on the report.
Batch Testing Protocols
Batch testing is at the heart of the Modern Aminos Quality Assurance Program.
Each vial, bottle, or blend is assigned a unique batch number and a scannable QR code linking to its verified lab report.
This system provides two easy ways to confirm authenticity:
- Scan the QR Code on your product label to access batch-specific testing results.
- Enter the Batch Number on our website to retrieve the official COA.
You’ll see the same batch number printed on both the COA and the vial photo, confirming the product in your hands matches the independently tested batch.
This process guarantees:
Full transparency in quality control
Verified third-party testing before release
Confidence that every research material is pure, consistent, and authentic
Types of Testing Conducted
At Modern Aminos, our commitment to quality begins long before a product reaches a researcher’s hands. Every batch is tested using a combination of analytical methods designed to confirm identity, purity, stability, and safety.
It’s important to note that not every research chemical receives every single test. Certain compounds, especially those known to be problematic or prone to contamination (e.g., Methylene Blue), undergo additional, targeted screening to ensure accuracy and safety.
Below is a breakdown of the testing methodologies we use:
HPLC: Purity & Identification
High-Performance Liquid Chromatography (HPLC) separates compounds based on their retention time and UV absorption.
- Confirms compound identity
- Provides a quantitative purity percentage (typically 98%+ for our products)
- Detects impurities or degradation products
NMR Spectroscopy: Structural Verification
Nuclear Magnetic Resonance (NMR) analyzes the molecule’s behavior in a magnetic field, identifying unique resonance frequencies.
- Confirms molecular structure and chemical integrity
- Verifies the compound matches its intended chemical fingerprint
TLC: Purity & Presence Confirmation
Thin-Layer Chromatography (TLC) is a quick, effective method to visually compare compound migration patterns against reference standards.
- Confirms the presence of the correct compound
- Provides a baseline purity check
ICP-MS: Heavy Metal Screening
Inductively Coupled Plasma Mass Spectrometry (ICP-MS) is a high-sensitivity method capable of detecting heavy metals (arsenic, cadmium, mercury, lead, etc.) at parts-per-trillion levels.
- Ensures materials are free from toxic contaminants
- Special note: Compounds like Methylene Blue receive additional ICP-MS screening due to a high industry rate of heavy metal contamination.
MS: Mass Spectrometry (MALDI / ESI)
Mass Spectrometry (MS) provides precise molecular weight confirmation and can detect trace impurities at extremely low concentrations.
- Confirms compound identity and molecular mass with high precision
- Detects unknown or unintended impurities that may not be picked up by TLC or HPLC
- Especially useful for complex or sensitive molecules such as peptides and novel research chemicals.
Endotoxin Testing (LAL Assay)
For most peptides, we now include endotoxin testing using the Limulus Amebocyte Lysate (LAL) assay.
- Detects pyrogenic (fever-causing) endotoxins from gram-negative bacteria
- Helps ensure product cleanliness and safety for research use
- Results are expressed in EU/mL (Endotoxin Units per mL)
Sterility Testing (Test Point Labs – Michigan)
Sterility is crucial for amino blends and pre-mixed solutions, as these formulations are more susceptible to microbial contamination.
We partner with Test Point Laboratories (Michigan), a certified microbial testing facility.
Each batch is sampled and tested for:
- Bacteria
- Yeast
- Mold
All results must show no microbial growth before approval. This ensures the product is clean, stable, and reliable for research purposes.
Summary:
- Not every compound undergoes every test, some receive extra targeted screenings to address known industry risks.
- Our layered testing approach provides a multi-angle verification of identity, purity, and safety.
- This commitment ensures researchers receive material they can trust with confidence.
Additional Testing Protocols
- Degradation Testing: Applied to sensitive compounds (e.g., NAD+) to confirm chemical stability and expected shelf life.
- Ongoing Quality Audits: Randomized sampling of stored inventory to ensure consistency and purity over time.
Our Guarantee
At Modern Aminos, our guarantee is simple:
Every batch we sell must be independently tested, and follow the parameters of our Quality Assurance Program, no exceptions.
If you ever discover that a product was shipped without valid third-party testing, we will replace it immediately, free of charge.
This guarantee reflects our core belief:
Transparency and scientific rigor are not optional, they’re the standard
Thank you guys for researching with us!
- The Modern Aminos Quality Control Team!


